When the effects and risks of a new treatment are examined, "adverse events" are a larger framework than side effects.
Our study group asks patients to submit any worsening of their health condition that occurred after transplantation, along with a discussion of the causal relationship to the transplant, after all transplants and at the end of the post-transplant observation period. (See endpoints and causal relationship below)
If necessary, the condition of the donor stool used may be checked or retested.
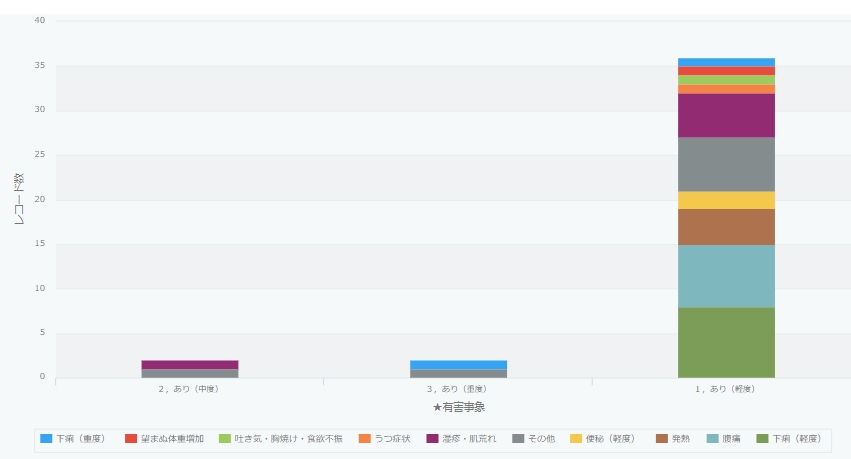
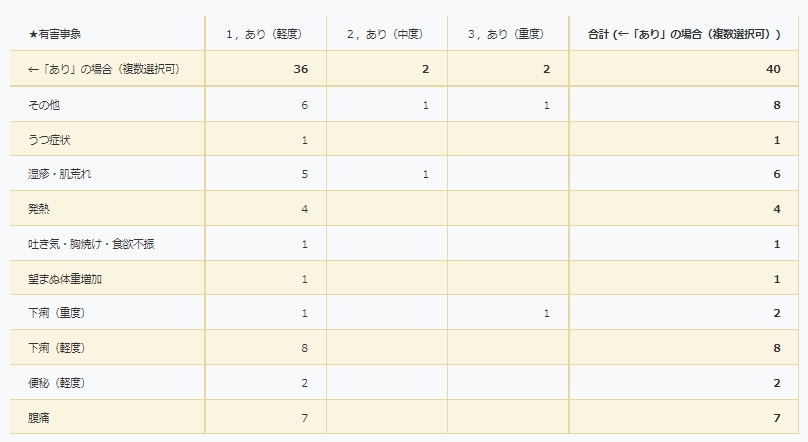
Adverse events of transplants performed in our study group
Adverse event endpoints (in accordance with the U.S. National Institutes of Health)
- Nothing
- Yes (mild): No hindrance to daily living
- Yes (moderate): Interferes with daily life
- Yes (severe): Requires emergency surgery or hospitalization. Includes cases of permanent and serious disability.
Adverse events are reflected after the completion of post-transplant follow-up. (Updated December 6, 2022)
Causal relationship with transplantation (in accordance with the U.S. National Institutes of Health)
- No causation: other causes, mismatch in timing, biologically improbable
- Causality unlikely: other causes, timing mismatch
- Possibly causal:indistinguishable from other factors
- Probably causal: if there is a reasonable temporal relationship and no other factors
- Clearly causally related: clear evidence is presented and other factors can be completely ruled out
Domestic and international information
Serious adverse events due to FMT are addressed in the “Report on Bacterial Products Based on Microbiome Research” from the Pharmaceuticals and Medical Devices Agency. The following is based on it.
No serious adverse events have been reported in Japan, but two serious adverse events (one death and one recovery) were reported in the United States in 2019.
In March 2019, two patients at Massachusetts General Hospital (MGH) who received FMT oral capsules of the same donor origin that were produced in-hospital were reported to have had ESBL (Extended spectrum β-lactamases) One patient received FMT to treat hepatic encephalopathy due to hepatitis C, but was found to be infected with ESBL. The patient recovered with antimicrobial therapy. Another patient undergoing hematopoietic stem cell transplantation to treat myelodysplastic syndrome (after cyclophosphamide and mycophenolate mofetil) underwent FMT to prevent graft-versus-host disease (GVHD). The patient died of severe sepsis due to infection with ESBL-producing bacteria after HSCT. The FDA promptly issued an alert and halted the provision of FMT nationwide.
An investigation revealed that MGH had not tested for ESBLs as required by FDA guidelines and that the donor stool was contaminated with ESBL-producing bacteria. IND testing using FMT was resumed with a recommendation to conduct thorough testing.
In addition, in 2020, 6 serious adverse events have been reported in 6 patients (all recovered); the FDA has issued an alert for this event as an adverse event due to enteropathogenic E. coli and Shiga toxin-producing E. coli based on donor stool.
Thus, the most important safety consideration for FMT is its potential to cause serious infections. For example, COVID-19 was noted early in the pandemic as a potential source of infection, as the virus was detected in stools; the FDA issued a safety alert in March 2020, and a new alert was issued on December 1, 2019 requested COVID-19 transmission prevention measures (testing of donors for infection and obtaining additional consent from recipients) when providing FMT stool juice using stools obtained on or after December 1, 2019.
The FDA has taken into account the FMT Infectious disease tests that should be considered, as suggested in the review on FMT, include tests for pathogenic bacteria, fungi, viruses, and parasites, but in our country, hepatitis E virus and others should be considered as well.
(Updated September 20, 2022)





