A Clinical Laboratory Technician Talks about the Stool Bank Japanbiome [Contents of Presentation at the 3rd Annual Meeting].
On September 23, 2019, the 3rd General Meeting of the Gut Flora Transplantation Clinical Research Society “A Letter to Humanity from Gut Bacteria” was held.
In this issue, we will report on the presentation on “Japanbiome (stool bank)” by Yoichiro Oka, a senior researcher at the institute!
Please see the presentation about Japanbiome (stool bank) at around 10:20 minutes 20 seconds into the video.
Establishment of Japanbiome (stool bank)
Japanbiome was born in July 2019. It will be a fecal version of the Red Cross Blood Center, which you all know and love, that conducts blood donations and transfusions.
In this issue, we will talk about safety in particular, not just what the test items are, but an overview of why they are necessary.
Japanbiome is a fecal banking facility for donors for intestinal flora transplantation. We only accept fecal donations that have passed our strict testing criteria.
Our philosophy (or motto) is to provide safe and secure intestinal flora transplants that are familiar to you and to take into account the characteristics of the donor stool at the time of donation in our selection. For detailed operation and testing, please visit the website of the Intestinal Flora Transplant Clinical Study Group (external site).
WHY IS FMT CALLED A TRANSPLANT?

Now, I mentioned earlier that we are an institution for intestinal flora transplantation, and I would like to review the transplantation that is currently taking place.
Next are tissue transplants such as heart valves, blood vessels, skin, and corneas, bone and bone marrow transplants, cell transplants using ips cells, which have made remarkable progress in recent years, and blood transfusions, which can also be called transplants.
All of these are recognized as medical treatment.
On the other hand, the feces we deal with is also a transplant in that it is donated from a donor to a recipient, and this is called a fecal microflora transplant (FMT).
However, FMT falls outside the medical category.
Differences from other “transplant” treatments
SO WHAT MAKES FMT DIFFERENT FROM OTHER TRANSPLANTS?
FIRST, OTHER TRANSPLANT MATERIALS ARE NECESSARY FOR HUMAN ACTIVITY IN THE FIRST PLACE. FECES, HOWEVER, ARE EXCREMENT. NORMALLY, FECES IS AN UNWANTED MATERIAL, BUT IN FMT, IT IS UTILIZED.
Second, in most cases, other transplant materials are collected and removed by medical personnel. Blood donation is also performed by medical personnel in mobile vans and blood donation rooms.
In addition, the collection and removal of blood is done in an appropriate environment (clean environment).
On the other hand, fecal collection is done by the donor himself/herself, with no medical personnel monitoring the defecation. Fecal collection is also done at each donor’s home.
THIRD, MOST OTHER TRANSPLANTS REQUIRE THE TRANSPLANT MATERIAL ITSELF TO BE STERILE BECAUSE THE MATERIAL IS IMPLANTED IN A STERILE PART OF THE BODY. FMT, ON THE OTHER HAND, REQUIRES VIABLE BACTERIA IN THE FECES.
FOURTH, AS I MENTIONED EARLIER, TRANSPLANTS OTHER THAN FMT ARE SOCIALLY ACCEPTED AS MEDICAL TREATMENT. FMT, ON THE OTHER HAND, IS OUTSIDE THE SCOPE OF LEGAL MAINTENANCE.
WHAT IS REQUIRED FOR FMT?
BASED ON THESE FOUR POINTS, THERE ARE THREE MAIN THINGS THAT ARE REQUIRED OF AN FMT.
First is strict donor selection.
As mentioned earlier, stool collection must be done by the donors themselves, and they must provide a stable supply.
Second, the fecal material provided is not sterilized, which increases the risk of infection. Therefore, management of fecal matter with increased safety is necessary.
Finally, there is traceability.
The donated stools are slightly modified to become a product as transplantation bacteria solution, which is then transplanted into the patient.
Therefore, it is necessary to have controls in place to track the feces used from the donor to the bank and then to the recipient.
Donor Selection
Now let’s delve a little deeper into the requirements we are looking for.
The first step is donor registration.
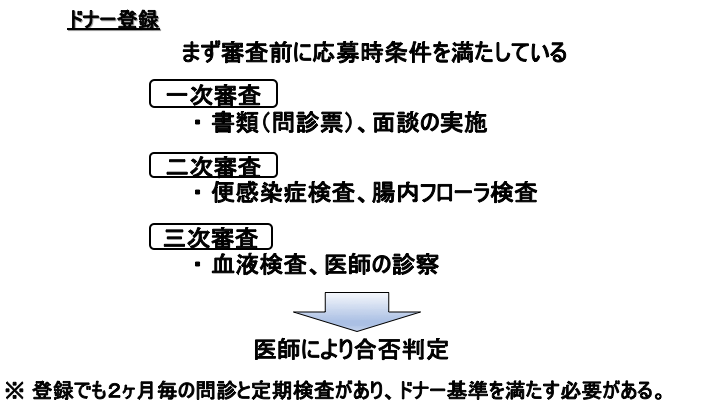
Donors must first meet the application requirements before being considered.
For example, no history of cancer or autoimmune disease.
Or, they must not be undergoing treatment for an infectious disease.
If you meet the pre-screening requirements, you will go through the first round of interviews and questionnaires.
If you pass the first round, you will go through the second round, fecal infection testing and intestinal flora analysis testing.
If you pass the second round, you will go through the final screening, blood tests and physician consultation.
The doctor will then make a comprehensive decision on whether or not you are eligible to register as a donor.
However, even after registration, the donor must still undergo a bi-monthly medical interview and periodic examinations and must meet donor criteria.
Ensuring the safety of donor stools
Next is donor stool safety.
This is the most important aspect of infection control.
A questionnaire regarding infectious disease-related issues must be attached to each submission of fecal material from donors who have reached registration.
Next, when the stools arrive at the bank, they are observed visually (Bristol Scale, etc.). (Bristol Scale, etc.) In addition, stools supplied for transplantation are tested (blood and stools) approximately every 2 months, and only stools that have passed the tests before and after the test are used.
(For example, if the tests on 4/1 and 6/1 both pass, only stools submitted during this period will be used. Therefore, stools submitted after 6/1 will wait for the inspection on 8/1 to be used.)
Finally, we would like to discuss the shipping of the transplantation fungal solution from the bank.
The transplantation solution will be packed in substantial packaging, but it must remain unopened, of course, until it reaches the supplier.
Therefore, a security seal is attached to the packing bag of the transplanted bacteria solution.
In addition, transportation is done by courier service, and temperature control during transportation is also important. The bank monitors the temperature control by placing irreversible temperature control stickers on the bags of transplanted bacteria solution as a spot check.
Here is an example of infection control. The report on this slide was issued by the United Nations.
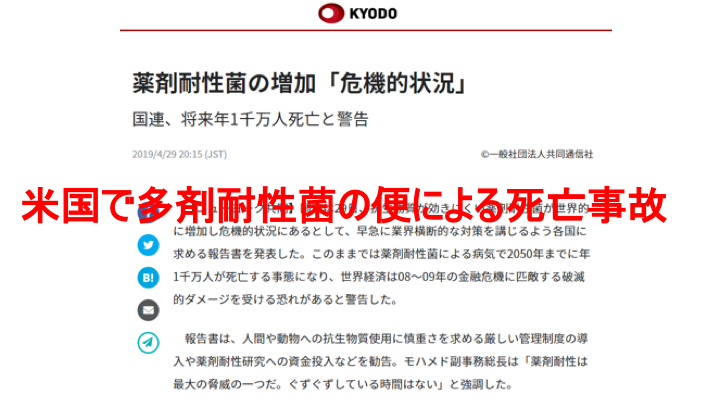
In recent years, drug-resistant bacteria have become a growing problem due to the overuse of antibiotics. Reports warn that as many as 10 million people a year could die from drug-resistant infections in 2050. Drug-resistant bacteria can be carried by healthy people, and it is impossible to know if they are carrying the bacteria until they are tested.
On the other hand, if an immunocompromised person is infected with resistant bacteria, the antibiotics available may be limited or ineffective, and in the worst case, death may result.
This past June, there was a death in the U.S. in which a stool that had multidrug-resistant bacteria was transplanted into an immunocompromised patient. The cause was failure to test the donated stool for resistant bacteria.
It must have been quite hot this summer. I am also sure that you have seen many foreigners visiting Japan recently. Also, the Olympics are coming up next year in Tokyo. As Japan’s climate becomes hotter, it becomes easier for alien species that bring infectious diseases, which could only live in warm places, to survive. In addition, the global flow of people to and from Japan may spread infectious diseases that have been staying in non-specific places.
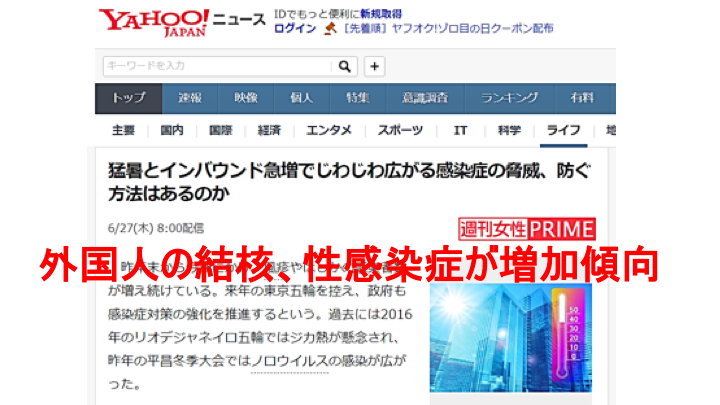
In fact, tuberculosis among foreigners visiting Japan has been on the increase in recent years. In addition, sexually transmitted diseases are on the increase in Japan.
Therefore, we need to take a hard look at fecal infection control in the future.
traceability
Next, we will discuss the traceability of fecal material.
Each donor, donor stool, and transplant stool source solution has its own ID number, and we have a system in place to keep track of each of the following: fecal donation, fecal storage and bacterial solution preparation, and bacterial solution supply.
We also keep a portion of each serum and stool so that we can conduct a retrospective survey when a problem arises.
This table will summarize what we have discussed so far about the flow of the donor stool safety system. You will notice that a lot of packaging and wrapping is involved in the collection of the fecal material and the shipment of the bacterial solution.
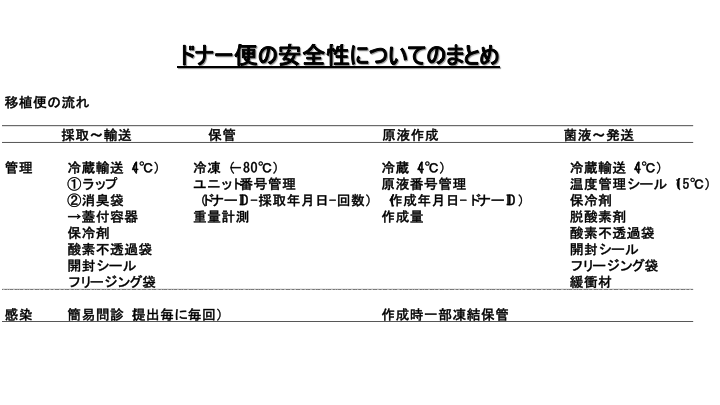
During transport at the time of collection, fecal material is wrapped in plastic wrap, deodorant bags, and placed in a covered container, which is then further wrapped in an oxygen-impermeable bag with a coolant and placed in a freezer bag with an opening seal. The received fecal material is weighed and stored at -80°C.
For shipment of the bacteria solution, the bags are sealed with a temperature control sticker when necessary, then wrapped in an oxygen-impermeable bag with oxygen absorber, and sealed with an opening seal. The bag is then placed in a freezer bag.
This slide is a photo of the donor mail upon arrival. It is sent by courier.
The fecal matter is wrapped in cushioning material and a questionnaire for infection-related matters is enclosed.
The fecal matter is contained in a container with a lid.

This slide shows a picture of the packaging of the fungal solution. It is hard to see, but a temperature control seal is attached to the bacteria solution bag.
We pack the bags with cushioning material and enclose a guide to the temperature control seal when we affix it, so that the customer can contact us if there are any abnormalities.
The rightmost one is the condition before shipping.

THIS IS AN OVERVIEW OF THE STOOL BANK OPERATION. THE BASIC SYSTEM FOR SECURING SAFE DONOR STOOLS AND SUPPLYING THEM IN THE FORM OF BACTERIAL SOLUTION IS NOW IN PLACE. ON THE OTHER HAND, WE HAVE NOT YET FULLY VERIFIED THE QUALITY AND EFFECTIVENESS OF THE TRANSPLANTATION SOLUTION, AND WE WILL CONTINUE OUR EFFORTS TO MAKE FMT MORE ACCESSIBLE TO THE PUBLIC.
Please see the presentation about Japanbiome (stool bank) at around 10:20 minutes 20 seconds into the video.
その他の Blog に関する記事
Articles about other Blog

October 26, 2021
WHAT IS THE GOAL OF FMT? IMPACT OF NUMBER OF TRANSPLANTS AND PRIOR ANTIBIOTIC ADMINISTRATION ON BACTERIAL VIABILITY
Effect of Number of Transplants and Prior Antibiotic Administration on Bacterial Engraftment Original Microorganisms | Free Full-Text | Engraftment of Bacteria after Fecal Microbiota Tra […].

August 03, 2021
Timing of intestinal bacteria to the inside of the mucosa
Conventional wisdom is that intestinal bacteria are only found in the outer mucus layer. The intestine is a hose-like cavity that serves as a pathway for bacteria, food, and water. The inside of the hose is not suddenly the cells of the large intestine, but the mucus and mucous layer separates the outside from the inside. The mucus layer […].
May 31, 2021
FMT IS EFFECTIVE AGAINST PARKINSON’S DISEASE AND RESISTANT BACTERIA [2021.5.31].
FMT relieves symptoms of Parkinson’s disease (Original) Evaluation of fecal microbiota transplantation in Parkinson’s disease patients wit […].




